Loading history...
Oxidation state in compounds
The oxidation state is a number that represents the electrical charge that an atom would have if the electrons shared in bonds were assigned to the more electronegative element. It is fundamental for understanding compound formation and their nomenclature.
Although in reality there is no complete transfer of electrons, it is considered that elements exchange electrons, with some left with positive charge.
The oxidation state is represented by an integer that can be positive, negative or zero. For example, in sodium chloride (NaCl), sodium has an oxidation state of +1 and chlorine -1. The most electronegative element becomes negatively charged and the least electronegative positively.
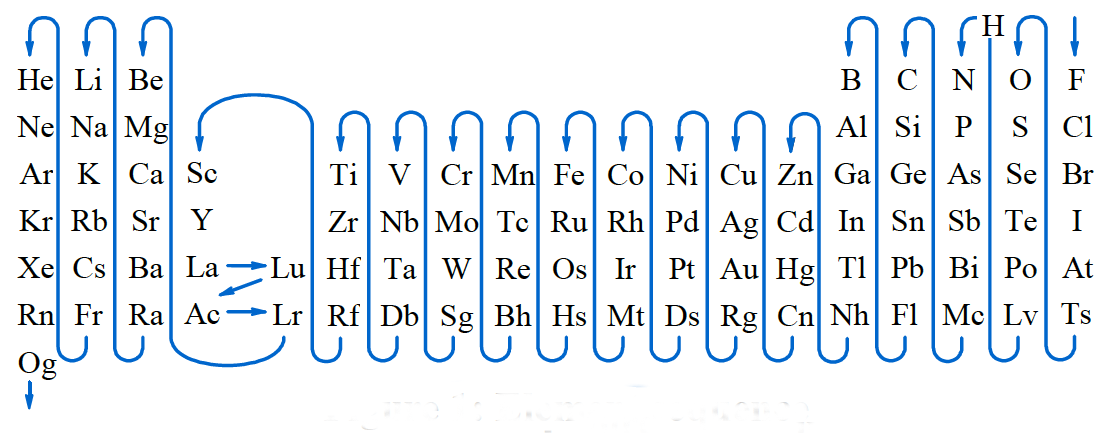
Whether an element loses or gains electrons depends on its electronegativity and its position in the periodic table. For this, fluorine is the most electronegative element, and electronegativity decreases when going down the periodic table and going to the left. Hydrogen is placed before nitrogen.
Chemical formulas
The chemical formula of an inorganic compound can be written in several ways.
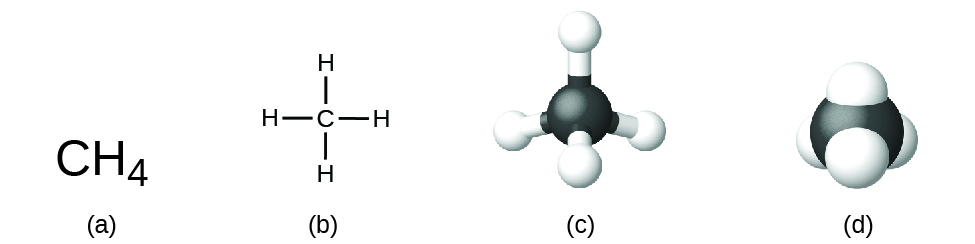
- Molecular formula: also called condensed. It consists of putting the elements of a compound in order of electronegativity, from least to greatest, and adding the number of each element using subscripts. For example, in methane (CH₄), carbon is less electronegative than hydrogen, so it is written first.
- Empirical formula: represents the simplest ratio between the elements. It is the first thing obtained when analyzing a compound by different traditional techniques. mass spectrometry. For example ethane (C₂H₆) has the empirical formula CH₃ which would be a rather unstable compound.
- Structural formula: shows the real arrangement of atoms in the molecule and how they are connected through bonds. It can be represented in various ways, from two-dimensional diagrams showing bonds to three-dimensional models that reflect the spatial geometry of the molecule. In turn, the structural formula can be semi-developed and only indicate part of the structure of the compound.
Valence number
The valence) number is the number of bonds or charge that an element usually has. Thus, according to their electronegativity, metals tend to lose electrons and acquire positive charge, while non-metals tend to gain electrons and acquire negative charge. Chemical elements tend to have the electronic configuration of noble gases, especially the elements closest to them. Elements furthest from noble gases acquire other electronic configurations since otherwise they would be excessively charged.
Most probable charge of an element
Each element tends to adopt certain oxidation states according to its position in the periodic table:
- Group 1 (alkali): +1
- Group 2 (alkaline earth): +2
- Group 13 (B, Al, etc.): +3
- Group 14 (C, Si, etc.): +4, -4
- Group 15 (N, P, etc.): -3
- Group 16 (O, S, etc.): -2
- Group 17 (halogens): -1

Creating neutral formulas
Chemical compounds are neutral, which means that the sum of the charges of the oxidation states of all atoms is zero. To create the formula of a compound, you must follow these steps:
- Determine the oxidation state of each element.
- Put the charge of the opposite element as the subscript of each element.
- Simplify the subscripts
Example: Al³⁺ + O²⁻ → Al₂O₃ (cannot be simplified further). Charge Al: +3·2 = +6; O: -2·3 = -6.
Example: S²⁺ + O²⁻ → S₂O₂ → SO. Charge S: +2·1 = +2; O: -2·1 = -2.
Inorganic nomenclature
Inorganic nomenclature refers to the way of naming compounds that do not consist of carbon chains, which give rise to life. Carbon's ability to bond in different ways makes it so that historically the chemistry of life and the elements created in the laboratory have been separated. Today we are already capable of studying all compounds in the laboratory but this differentiation continues to be maintained. In turn, other nomenclatures such as that of polymers have been developed.
The IUPAC (International Union of Pure and Applied Chemistry) is the organization that establishes attempts to establish a regular method for naming compounds so that they have a unique name and allow their structure to be known.
Due to the development of chemistry, its nomenclature has been changing and adapting to new discoveries and compounds. In the IUPAC blue book you can find the rules for naming this type of compounds that you can consult in summary form in the IUPAC brief guide